Fourth dose COVID vaccine
No new safety concerns. While protection from a third COVID-19 vaccine dose seems to wane after four months experts arent so sure that a fourth dose will be necessary for all.

Fourth Dose Of Covid Vaccine Offers Only Slight Boost Against Omicron Infection
Ad For a full list of ingredients please see the CDC fact sheet for each COVID vaccine.
. ATAGI expands COVID-19 booster access to allow more people to get a fourth dose. Access expands for fourth covid vaccine doseA FURTHER 15 million Australians will be eligible for a fourth dose of a COVID 19 vaccine from May 30. The third dose is administered at least 4 weeks after.
People ages 12 years and older who are moderately or severely immunocompromised should receive a total of 4 doses of mRNA COVID-19 vaccine to stay up. In this open-label nonrandomized clinical study we assessed the immunogenicity and safety of a fourth dose of either BNT162b2 PfizerBioNTech or mRNA-1273 Moderna. Scientists in Israel also report that a fourth vaccine dose.
None of the authorized or approved vaccines contain the live virus that causes COVID. The Australian Technical Advisory Group on Immunisation ATAGI has recommended an additional vaccine dose for the following groups. A fourth dose of the BNT162b2 vaccine was effective in reducing the short-term risk of Covid-19related outcomes among persons who had received a third dose at least 4.
Health regulators on Tuesday cleared fourth Covid vaccine doses for older adults amid uncertainty over whether an. FDA authorizes fourth Covid vaccine dose for people age 50 and older The top US. Fourth Dose of BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting N Engl J Med.
Compared with a third vaccine dose a fourth dose of the PfizerBioNTech COVID-19 vaccine lowered the risk of infection symptomatic infection hospitalization severe illness and death 52 to 76depending on the measureamid the Omicron surge among older adults finds a new Israeli study. Online ahead of print. Only the Pfizer vaccine can be used in those as young as 12.
FDA authorizes fourth Covid vaccine dose for people age 50 and older Rather than saying that the protection wanes I would say that this boost effect is strongest shortly. Pfizer-BioNTech COVID-19 Vaccine 5 years and older. Many variants are coming and Omicron.
AAP The Australian Technical Advisory Group on. Fourth dose is a booster shot Also in August the Food and Drug Administration FDA authorized the third additional vaccine dose for people who are immunocompromised. Who can get a fourth COVID jab.
Authors Ori Magen 1 Jacob G. Fourth dose of COVID vaccine offers only slight boost against Omicron infection Israeli trial shows a fourth vaccination raises antibody levels but provides little extra protection. The fourth dose increased neutralizing antibody levels against the coronavirus compared to the levels people had 5 months after the first booster.
People eligible for the fourth dose will be able to start getting it by next week. Tue 24 May 2022 717am. Centers for Disease Control CDC provided guidance stating that immunocompromised individuals could receive a booster shot a fourth dose six months.
Until now the FDA had allowed a fourth vaccine dose only for the immune-compromised as young as 12. Originally the US. To help fend off another wave of Covid-19 people will need a fourth dose of vaccine Pfizer CEO Albert Bourla told CBS on Sunday.
Immunocompromised People Should Get a Fourth Shot The CDC recommends that moderately or severely immunocompromised individuals who received a two-dose mRNA. An expanded group of people is now eligible for a fourth dose to be given four months after the initial booster. The 4th dose or 2nd booster COVID-19 vaccines train the bodys immune system to elicit a rapid response to SARS-CoV-2.
Lela Moore is a. Regulators in Europe say getting too many COVID-19 booster shots may actually weaken your immune response. Protection against infection waned however after 5 weeks but not.
A fourth dose of a COVID-19 mRNA vaccine can boost antibodies and other immune responses to levels higher than those seen after the third dose according to UK trial. The Government has set aside nearly half a billion dollars for purchasing and rolling out another dose of the Covid-19 vaccine and other Covid-19. The second dose is administered 3 weeks after the first dose.
They induce an immune response involving the activation of B cells which.
/cloudfront-us-east-1.images.arcpublishing.com/gray/NY6G5YPNTRBL7FKBLYD7OXLTGA.jpg)
Fact Finders Who Is Eligible For The Fourth Dose Of The Vaccine

Eu Members Question Fourth Dose Except Most Vulnerable Euractiv Com
/cloudfront-us-east-1.images.arcpublishing.com/gray/COIGUL3PCND75MUK3PX7C4UQGE.jpg)
Uva Doctor Explains Who Qualifies For A Fourth Dose Of The Covid Vaccine

Covid Vaccines It S Too Early To Consider Giving People A Fourth Dose Say Eu Officials Euronews

Pfizer Asks Us To Allow 4th Covid Vaccine Dose For Seniors Wear
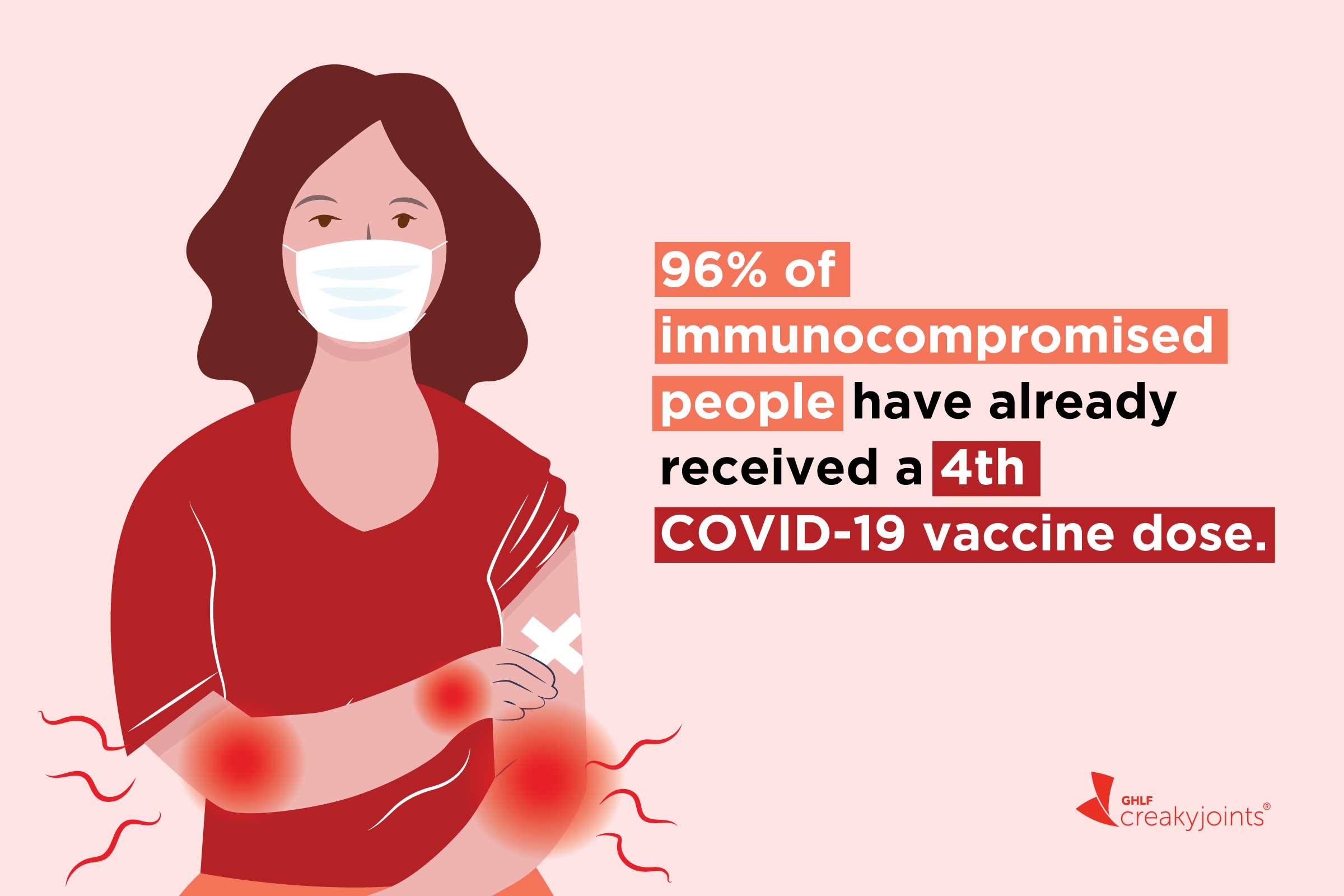
Most Immunocompromised People Have Gotten The 4th Covid 19 Vaccine Dose
Israel Mulls Offering 4th Covid Vaccine Dose To All Adults Reuters

Pfizer Asks Us To Allow 4th Covid Vaccine Dose For Seniors

Cdc Some Immunocompromised People Can Get A Fourth Dose Medpage Today
Fourth Doses Of Covid 19 Vaccine Available Thursday For 60 And First Nations My Bancroft Now
Fourth Dose Of Covid 19 Vaccine Reduced Death Among Hospitalized Patients During Omicron Wave In Israel
How Effective Is A Fourth Dose Of Covid 19 Vaccine Gavi The Vaccine Alliance

With Covid Cases Surging Across Australia Will A Fourth Vaccine Dose Be Required Coronavirus The Guardian

Italy Recommends Fourth Dose For Immunocompromised Patients Euractiv Com

Israeli Trial World S First Finds 4th Dose Not Good Enough Against Omicron The Times Of Israel

Coronavirus Digest Fourth Jab Not Enough To Fight Omicron Study Shows News Dw 17 01 2022